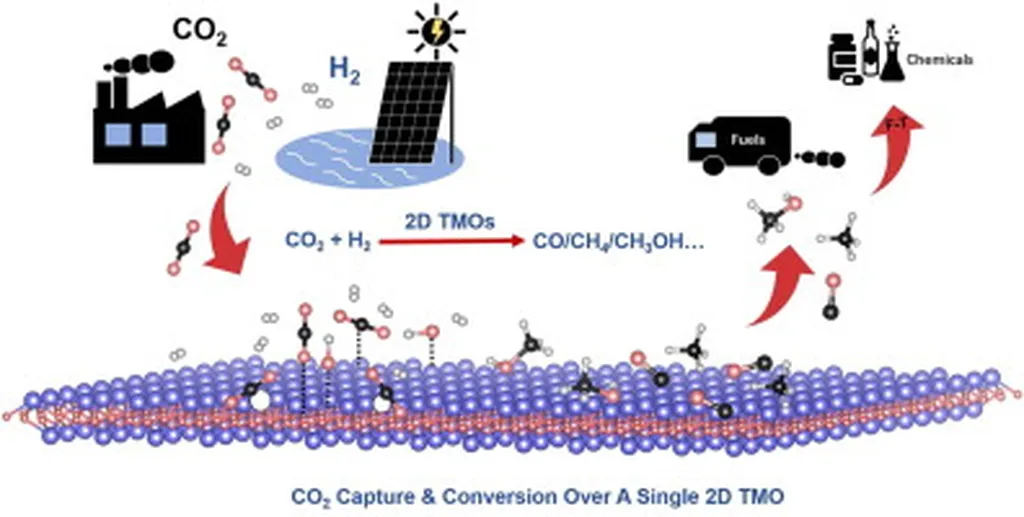
In a significant stride towards combating climate change, researchers have developed novel binary metal oxides that could revolutionize carbon capture and utilization (CCU) technologies. Published in the journal Nature Scientific Reports, the study explores the potential of Ti-Zr, Ti-Ce, and Zr-Ce oxides as dual-function materials for CO₂ adsorption and ethylene urea (EU) synthesis. This breakthrough could pave the way for more efficient and cost-effective methods of converting captured CO₂ into valuable chemicals, a critical aspect of the energy sector’s decarbonization efforts.
The research, led by Farzana Rahman from the Graduate School of Environmental Studies at Tohoku University, focuses on the synthesis and application of binary metal oxides. These materials were prepared using sol–gel and solvothermal methods, resulting in varying crystallinities and surface areas. Notably, the TiₓZr₍₁₋ₓ₎O₂ oxides exhibited the highest surface area, approximately 150 m²/g, which significantly enhanced their CO₂ adsorption capabilities.
“Our findings demonstrate that TiₓZr₍₁₋ₓ₎O₂ oxides, particularly Ti₀.₃Zr₀.₇O₂, show great promise as adsorbents and reaction accelerators,” Rahman explained. “The high surface area and CO₂ adsorption capacity of these materials make them highly effective for capturing and utilizing CO₂ in the synthesis of ethylene urea.”
The study revealed that CO₂ adsorption on these binary oxides followed the Langmuir and Freundlich models, indicating a strong affinity for CO₂ molecules. When heated, the CO₂-loaded oxides facilitated the production of ethylene urea, with CeO₂ showing superior selectivity. The reaction mechanism involved CO₂ desorption and dehydration, highlighting the dual-functionality of these materials.
The commercial implications of this research are substantial. Ethylene urea is a valuable chemical used in various industries, including agriculture and textiles. By developing a more efficient and cost-effective method for its synthesis from captured CO₂, this research could drive the adoption of CCU technologies in the energy sector. The ability to convert CO₂ into useful chemicals not only reduces greenhouse gas emissions but also creates economic value, aligning with the principles of a circular economy.
“This research opens up new avenues for the energy sector to explore,” Rahman added. “By integrating these binary metal oxides into existing CCU technologies, we can enhance their efficiency and economic viability, ultimately contributing to a more sustainable future.”
The study’s findings underscore the potential of TiₓZr₍₁₋ₓ₎O₂ oxides as promising catalysts for CO₂ utilization and ethylene urea synthesis. As the world continues to seek innovative solutions to address climate change, this research offers a glimpse into the future of carbon capture and utilization technologies. By converting captured CO₂ into valuable chemicals, we can reduce emissions and create economic opportunities, paving the way for a more sustainable and prosperous future.