In a significant stride toward sustainable energy solutions, researchers have made notable advancements in the development of catalysts for the electrochemical reduction of carbon dioxide (CO2). This promising technology could revolutionize the energy sector by offering a carbon-neutral route for fuel and chemical production, potentially reducing reliance on fossil-based processes. The study, led by Lucija Josipovic, a student in the Class of Electrochemistry at New Mexico State University, was recently published in the journal “Inorganics,” which translates to “Inorganic Chemistry” in English.
The electrochemical CO2 reduction reaction (eCO2RR) stands out as a viable method for converting CO2 into valuable products under ambient conditions. Unlike conventional CO2 conversion techniques, which often require extreme temperatures and pressures, eCO2RR operates at room temperature and atmospheric pressure, making it more energy-efficient and environmentally friendly. “The electrochemical approach offers a sustainable alternative that can be integrated into existing energy systems, paving the way for a greener future,” Josipovic explained.
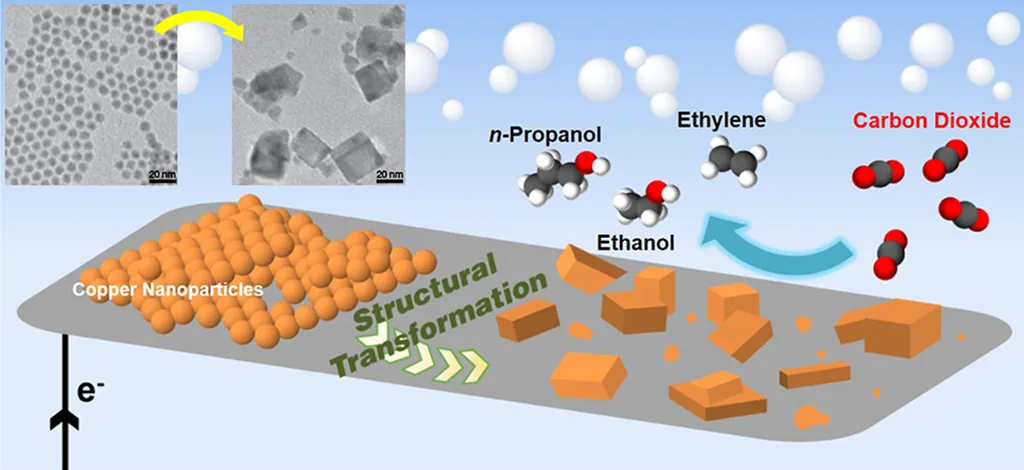
The research delves into recent advancements in electrocatalyst development, focusing on three main categories: metal-based, Cu-based, and metal-free catalysts. Metal-based catalysts are categorized by their product selectivity, with some catalysts excelling in the production of formate, others in CO, and a few in multicarbon products. “Understanding the structure and performance of these catalysts is crucial for optimizing the eCO2RR process,” Josipovic noted.
Cu-based catalysts are particularly noteworthy due to their unique ability to produce multicarbon products, which are highly valuable in the chemical industry. The study highlights various design strategies and material types that enhance the performance of Cu-based catalysts, offering insights into their potential applications.
Emerging metal-free catalysts, including their synthesis, mechanisms, and potential applications, are also reviewed. These catalysts present a promising alternative to traditional metal-based catalysts, offering advantages such as lower cost and higher durability.
The comparative analysis provided in the study aims to guide future research toward the development of more efficient, selective, and durable catalysts for CO2 electroreduction. “Our goal is to accelerate the deployment of carbon capture and utilization technologies, contributing to a more sustainable energy landscape,” Josipovic stated.
The implications of this research are far-reaching, with the potential to shape future developments in the energy sector. By enabling the conversion of CO2 into valuable products, eCO2RR technology could play a pivotal role in reducing greenhouse gas emissions and mitigating climate change. The study’s findings offer a roadmap for researchers and industry professionals, highlighting the most promising avenues for further exploration and development.
As the world grapples with the challenges of climate change and energy sustainability, innovations in eCO2RR technology offer a beacon of hope. With continued research and development, this technology could become a cornerstone of the global effort to transition to a low-carbon economy. The study published in “Inorganics” serves as a testament to the power of scientific inquiry and the potential for technological innovation to drive positive change in the energy sector.