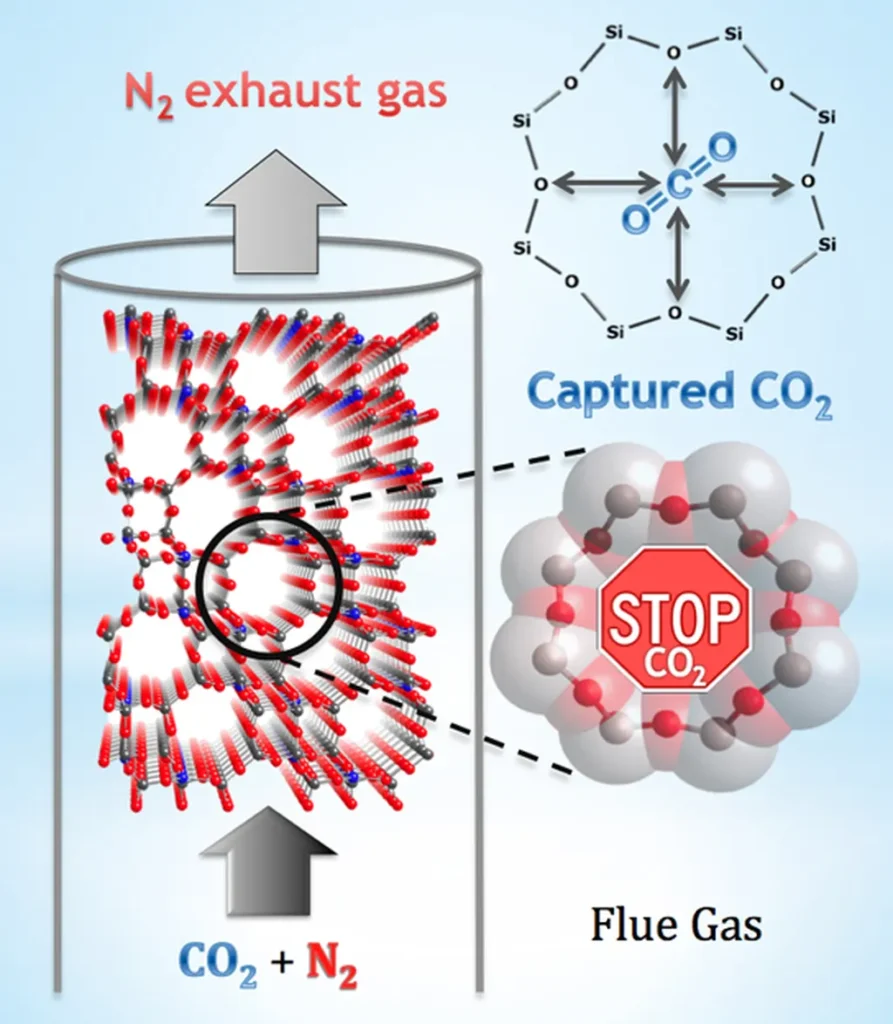
In a significant stride towards enhancing carbon capture technologies, researchers have unveiled intricate details about how carbon dioxide (CO₂) interacts with specific types of zeolites, a family of porous minerals widely used in industrial separations. The study, led by Magdy Abdelghany Elsayed from the College of Energy and Mining Engineering at Shandong University of Science and Technology in China, offers a comprehensive analysis of CO₂ adsorption and diffusion in LTA-type zeolites, providing valuable insights for the energy sector.
The research, published in the journal “Nanomaterials,” combines laboratory measurements with advanced computational techniques to shed light on the adsorption mechanisms of CO₂ in zeolites ITQ-29 and 5A. “Understanding these mechanisms is crucial for developing efficient technologies to capture CO₂ from industrial flue gases,” Elsayed explained. The findings reveal that the adsorption process is spontaneous and exothermic, with the enthalpy change and entropy change providing critical data for optimizing adsorption conditions.
One of the key discoveries is the variation in adsorption energy between the two zeolites. “CO₂ exhibited a significantly more negative adsorption energy in zeolite 5A compared to ITQ-29, due to strong interactions with Ca²⁺ and Na⁺ cations present in 5A,” Elsayed noted. This difference highlights the importance of cation presence in enhancing CO₂ adsorption, a factor that could guide the design of future adsorbents.
The study also delves into the kinetics of CO₂ adsorption, revealing that the process follows a pseudo-first-order model with an activation energy that confirms physisorption. The intraparticle diffusion model indicates that internal diffusion is the rate-limiting step, a finding that could influence the development of more efficient adsorption technologies.
Molecular dynamics simulations further illustrated that CO₂ diffuses more rapidly in ITQ-29 than in 5A, with diffusion coefficients and activation energies providing a quantitative measure of this behavior. “The good agreement between experimental measurements and molecular simulation results for zeolite 5A validates our simulation parameters, allowing us to study zeolite ITQ under similar conditions,” Elsayed added.
The implications of this research are far-reaching for the energy sector. By understanding the adsorption energetics and diffusion within LTA-type zeolite frameworks, researchers can design high-performance adsorbents tailored for industrial gas separation. This could lead to more efficient and cost-effective carbon capture technologies, crucial for reducing greenhouse gas emissions and mitigating climate change.
As the world grapples with the challenges of decarbonization, studies like this one provide a beacon of hope. By unraveling the complexities of CO₂ adsorption and diffusion, Elsayed and his team are paving the way for innovative solutions that could transform the energy landscape. Their work not only advances our scientific understanding but also offers practical insights for developing next-generation carbon capture technologies.