In the quest for safer, more efficient batteries, researchers have long been captivated by solid electrolytes, which promise to replace the flammable liquid electrolytes found in today’s lithium-ion batteries. A recent study published in Electrochemistry, the journal of the Electrochemical Society of Japan, has brought us a step closer to this goal. The research, led by Kazuhiro HIKIMA from the Department of Electrical and Electronic Information Engineering at Toyohashi University of Technology, introduces a rapid and scalable synthesis method for a promising solid electrolyte material.
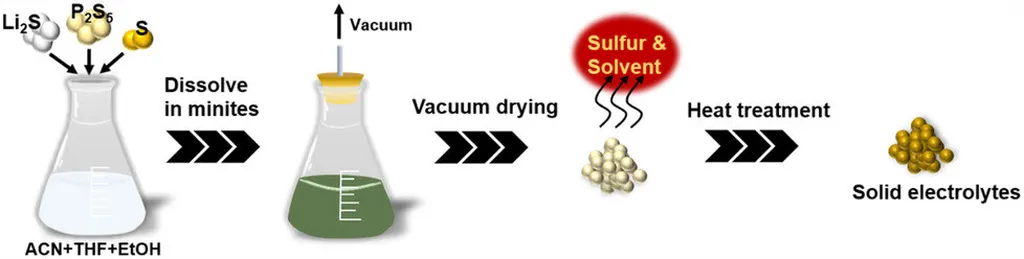
The material in question is a variant of the Li10GeP2S12 (LGPS) structure, with silicon, phosphorus, sulfur, and chlorine (LSiPSCl). This class of materials has garnered significant attention due to its high ionic conductivity, a crucial factor for battery performance. However, traditional synthesis methods, such as mechanical milling, can be time-consuming and challenging to scale up for mass production.
Hikima and his team have developed a solution-based method that addresses these issues. By using a mixture of acetonitrile, tetrahydrofuran, and ethanol as a solvent, along with excess sulfur, they were able to synthesize the LSiPSCl solid electrolyte rapidly and in large quantities. “The solution method allows for a more controlled and uniform reaction, which is beneficial for large-scale production,” HIKIMA explained.
The synthesized material exhibited an impressive ionic conductivity of 2.7 mS cm−1, comparable to that of samples made using the mechanical milling method. But the real test was in the battery. When incorporated into an all-solid-state battery, the LSiPSCl solid electrolyte synthesized via the solution method showed a slightly higher discharge capacity and similar cycle stability compared to its mechanically milled counterpart.
The team’s analysis revealed an intriguing factor contributing to this enhanced performance: a carbon surface layer on the particles, originating from the solvent. This surface layer, identified through Raman and X-ray photoelectron spectroscopy, seems to play a key role in improving the battery’s discharge capacity. “The surface layer and particle characteristics are critical advantages of the solution synthesis method,” HIKIMA noted. “They could be the key to further improving battery performance.”
This research opens up exciting possibilities for the energy sector. The solution synthesis method’s scalability and the enhanced performance of the resulting solid electrolyte could pave the way for more efficient, safer, and longer-lasting all-solid-state batteries. These batteries could revolutionize electric vehicles, grid storage, and other energy applications, contributing to a more sustainable and electrified future.
As we look ahead, it’s clear that the surface layer and particle characteristics highlighted in this study will be crucial areas of focus. Further research could unlock even greater performance improvements, bringing us closer to the next generation of battery technology. With the findings published in Electrochemistry, the journal of the Electrochemical Society of Japan, the scientific community now has a solid foundation to build upon, driving forward the development of all-solid-state batteries.