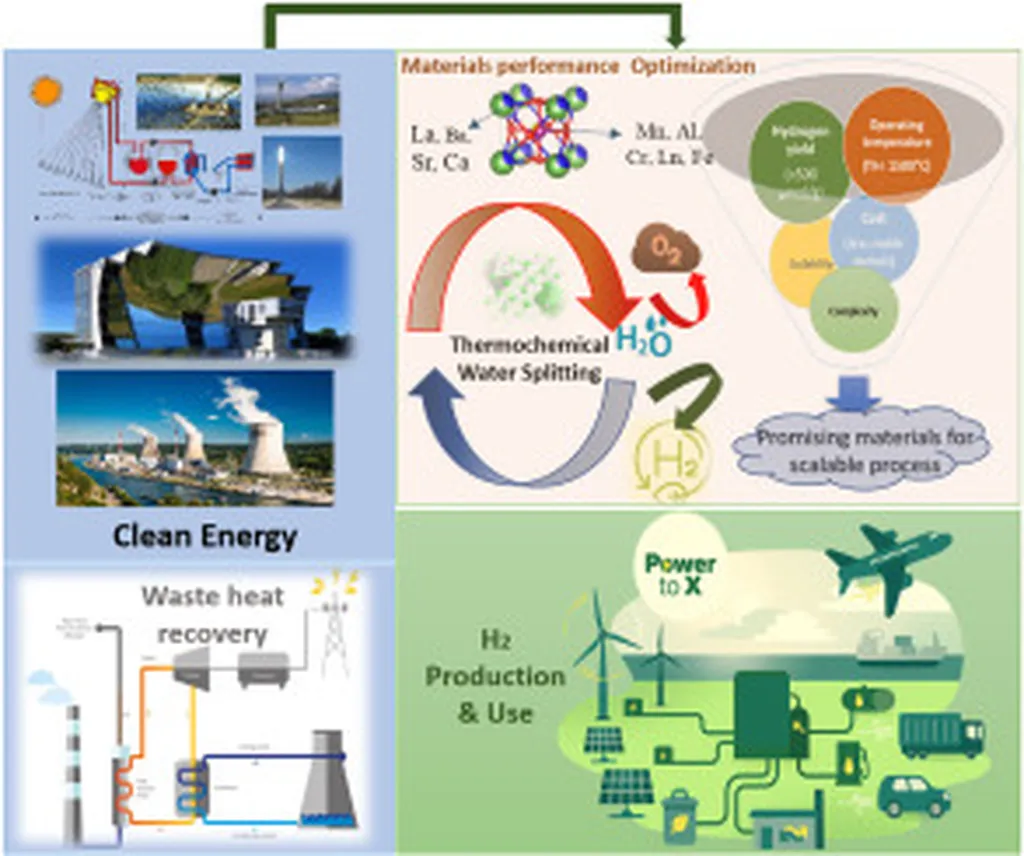
A team of researchers has achieved a landmark advancement in green hydrogen production, demonstrating a mechanocatalytic approach to thermochemical water splitting at room temperature. This breakthrough, detailed in a recent study, promises to revolutionize the efficiency and scalability of hydrogen generation, addressing one of the most persistent barriers to a sustainable hydrogen economy.
Traditionally, thermochemical water splitting—a process that uses heat and chemical reactions to break water into hydrogen and oxygen—has required high temperatures, often exceeding 1,000°C. Such extreme conditions demand significant energy input and specialized materials, limiting practical deployment. The new method, however, leverages mechanocatalysis, where mechanical force activates catalytic materials to split water at ambient temperatures. This approach not only slashes energy requirements but also opens the door to decentralized, on-demand hydrogen production.
The innovation, spearheaded by researchers at a leading Japanese institution, utilizes advanced materials such as ferrite and cerium-based oxides, which exhibit exceptional catalytic activity when subjected to mechanical stress. “This method achieves efficiencies of 40–50%, with the potential to exceed 60% as we optimize material compositions and reaction conditions,” explained Dr. Yamamoto, the study’s lead author. “By eliminating the need for high-temperature reactors, we can significantly reduce both capital and operational costs, making green hydrogen more accessible for industrial and transportation applications.”
This breakthrough directly challenges the status quo of hydrogen production, which has long relied on energy-intensive electrolysis or fossil fuel-derived processes. The ability to produce hydrogen at room temperature could accelerate the integration of green hydrogen into sectors such as steelmaking, aviation, and heavy transport, where decarbonization has been particularly stubborn. Moreover, the technology’s modular nature means it can be deployed at various scales, from small community projects to large industrial plants, without the need for extensive infrastructure overhauls.
The implications for global energy sustainability are profound. Green hydrogen, produced without carbon emissions, is increasingly seen as a cornerstone of the energy transition. This new method not only enhances the economic viability of hydrogen but also aligns with international climate goals by offering a scalable, low-carbon alternative to fossil fuels. As policymakers and industry leaders grapple with the urgency of reducing emissions, innovations like room-temperature thermochemical water splitting could be the catalyst needed to tip the balance toward a hydrogen-powered future.
With pilot projects already underway in Japan and Europe, the next phase will focus on scaling the technology and integrating it with renewable energy sources. If successful, this breakthrough could redefine the hydrogen landscape, making clean energy more affordable, efficient, and accessible than ever before.