In the realm of materials science, researchers Ksenia Khoroshun, Mario U. González-Rivas, and Alannah M. Hallas from the University of Waterloo are delving into the complexities of high entropy oxides (HEOs), materials that could potentially revolutionize the energy industry. Their work, published in the journal Nature Communications, aims to understand how the chemical composition of HEOs influences their stability, a crucial factor for practical applications.
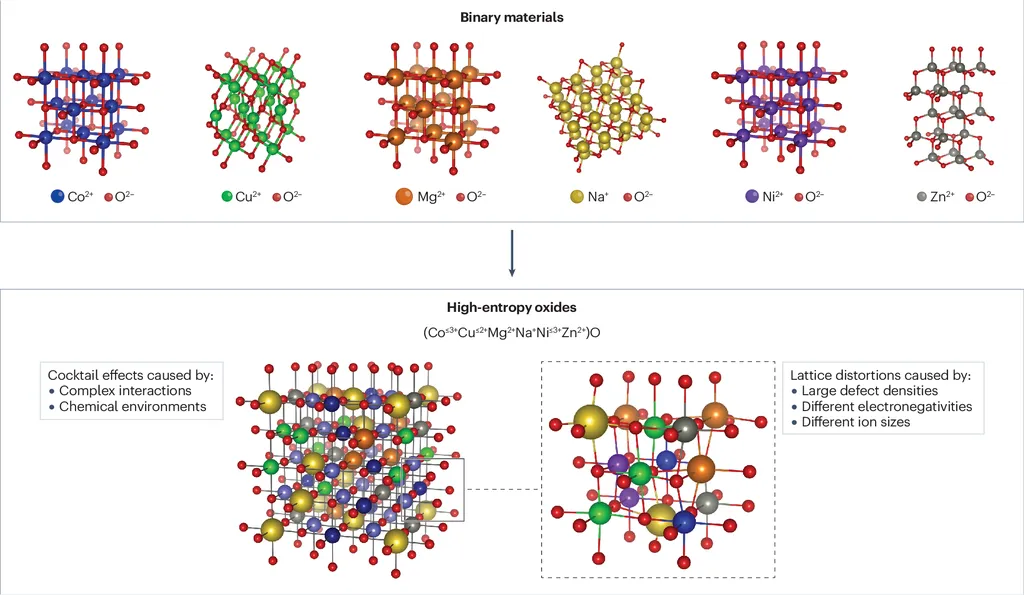
High entropy oxides are a class of materials that contain multiple elements in roughly equal proportions, leading to high configurational entropy. This entropy can enhance certain material properties, making HEOs promising candidates for various energy applications, such as more efficient catalysts, improved battery materials, and advanced fuel cells. However, synthesizing these materials is often challenging due to competing factors that affect their stability.
The researchers focused on four notable complex oxide families: perovskite, pyrochlore, Ruddlesden-Popper, and zirconium tungstate. Each of these structures has a specific site that the researchers attempted to substitute with an entropic mixture of four cations. They also synthesized non-disordered reference compounds for comparison.
Their findings revealed that while all four target high entropy materials could be expected to form based on ionic radii criteria, only the high entropy perovskite Ba(Ti,Zr,Hf,Sn)O3 was successfully synthesized. For the pyrochlore, an entropy-stabilized defect fluorite was formed instead. The Ruddlesden-Popper phase coexisted with multiple competing phases, and for the tungstate, an unexpected deep eutectic point between the precursors resulted in melting that preceded the formation of a high entropy phase.
These case studies illustrate that the stability of HEOs cannot be straightforwardly predicted based on ionic radii, lattice geometry, and charge-balancing considerations alone. The underlying complexity of the interactions between the many chemical constituents plays a significant role.
For the energy industry, this research highlights the potential of HEOs but also underscores the need for a deeper understanding of their behavior. As we strive for more efficient and sustainable energy solutions, materials like HEOs could play a pivotal role. However, their successful synthesis and application require a nuanced approach that accounts for the intricate interplay of their chemical constituents.
Source: Nature Communications
This article is based on research available at arXiv.