In a significant stride towards understanding and optimizing energy-related materials, researchers have uncovered intricate details about the structure and behavior of Sr and Mg doped lanthanum gallate single crystals. This work, led by Sergej Ražnjević from the Erich Schmid Institute of Materials Science at the Austrian Academy of Sciences, was recently published in the American Physical Society’s journal, which translates to ‘APL Materials’.
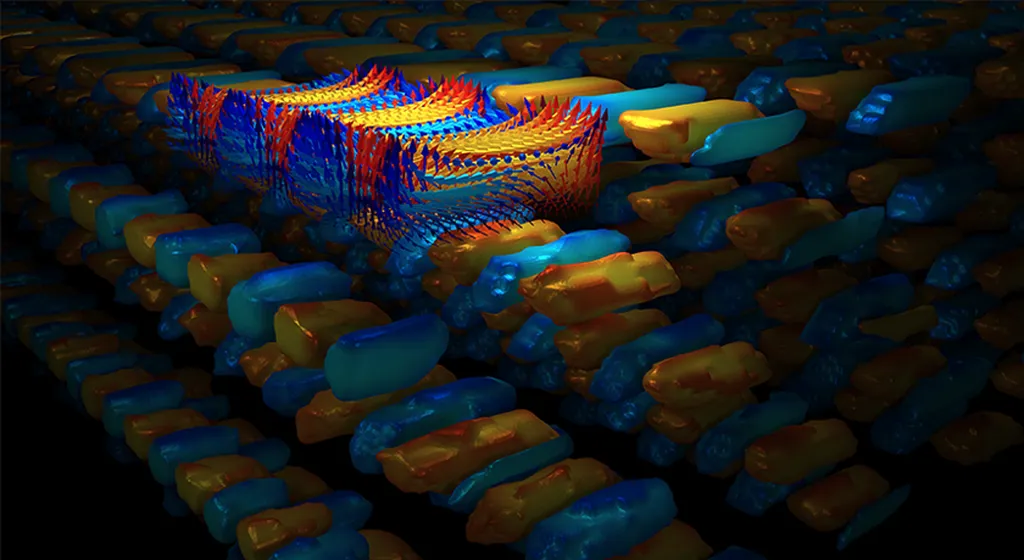
The study delves into the complex world of oxygen vacancies, which are crucial for the functionality of many energy materials, including solid oxide fuel cells and electrolysis cells. By employing advanced techniques such as x-ray diffraction (XRD) and transmission electron microscopy (TEM), Ražnjević and his team have revealed the presence of multiple domains within these crystals, each with distinct lattice parameters and oxygen vacancy distributions.
One of the most striking findings is the discovery of two distinct regions within the crystals: one exhibiting 90° symmetry and the other with non-90° symmetry. “The ordered oxygen vacancies in the non-90° region were particularly intriguing,” Ražnjević noted. “This level of organization is not typically seen and could have significant implications for the material’s properties and performance.”
The team also found that the oxygen vacancies in the 90° region were randomly distributed, highlighting the complex interplay between structure and functionality in these materials. Energy-dispersive x-ray spectroscopy confirmed that the difference in the doping level of the two regions was within the detectability limits, but the electron energy loss near-edge structure revealed detectable differences in their bonding character.
So, what does this mean for the energy sector? Understanding the distribution and behavior of oxygen vacancies is crucial for developing more efficient and durable energy materials. “This research provides a deeper insight into the fundamental properties of these materials,” Ražnjević explained. “By understanding these details, we can potentially tailor the materials to enhance their performance in energy applications.”
The findings could pave the way for the development of more efficient solid oxide fuel cells and electrolysis cells, which are key technologies for converting and storing energy. Moreover, the advanced characterization techniques employed in this study could be applied to a wide range of materials, opening up new avenues for research and development in the energy sector.
As we strive towards a more sustainable future, understanding and optimizing the materials that power our world becomes increasingly important. This research is a significant step in that direction, offering valuable insights that could shape the future of energy materials and technologies.